The Value of Serological Screening for Gastric Cancer
Gastric cancer is a global health burden, with high mortality rates due to late-stage diagnoses. However, recent advancements in medical research have led to the development of serological screening tests that had shown promising results in detecting gastric cancer at an early stage.
Serological screening involves analyzing blood samples to detect specific biomarkers associated with gastric cancer. A recent study has highlighted the potential value of measuring the levels of PGⅠ/PGⅡ/G-17 in screening for gastric cancer. These biomarkers, found in the stomach lining, have shown promising results in detecting early-stage gastric cancer.The study examined a large cohort of individuals and found that abnormal levels of PGⅠ/PGⅡ/G-17 were significantly associated with the presence of gastric cancer. Furthermore, the combination of these biomarkers demonstrated a high accuracy rate in identifying individuals at risk of developing gastric cancer.
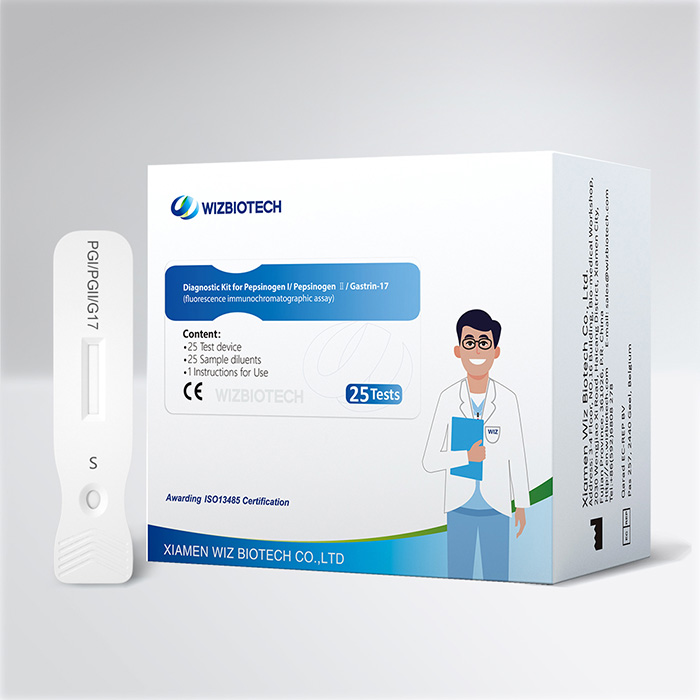
In conclusion, serological screening for gastric cancer has shown promising results in early detection, highlighting its value as a cost-effective and non-invasive tool. Diagnostic Kit of Pepsinogen I/Pepsinogen Ⅱ/Gastrin-17 (Fluorescence immunchromatographic assay), independently developed and produced by Xiamen Wiz Biotech Co., Ltd. is a low-cost testing project, where one reagent card completes the testing of three items simultaneously, truly achieves efficient and accurate joint detection.
Xiamen Wiz Biotech Co., Ltd is a high-tech company engages in the research and development, production and sales of rapid diagnostic reagents and instruments. Focusing on technological innovation, WIZBIOTECH develops POCT testing reagents and instruments, and takes advantage of existing channels to expand the medical market, and contributes more in the field of rapid diagnostic POCT.