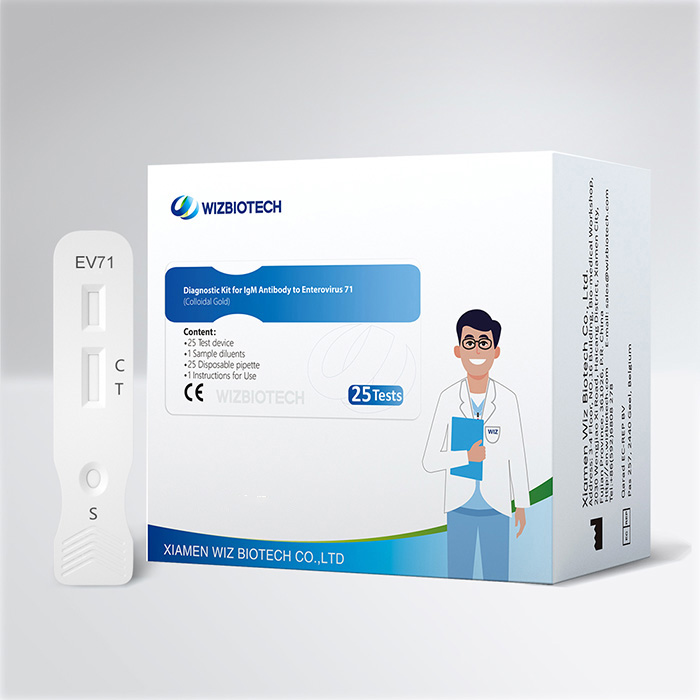
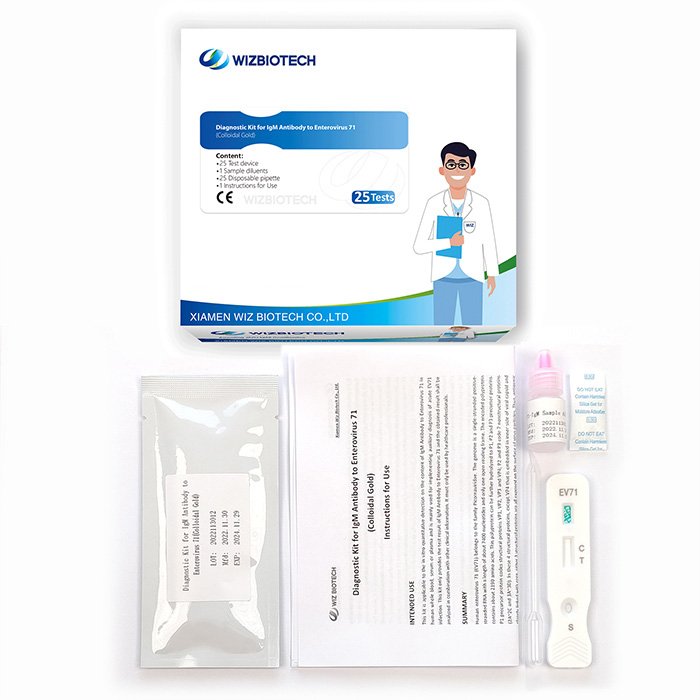
Lab Blood IgM Antibody To Enterovirus 71 Ev71 Test For Hfmd

- Wizbiotech
- CE, MDA
- China
This kit is applicable to the in vitro qualitative detection of IgM Antibody to Enterovirus 71 in human whole blood, serum or plasma. It only takes 10~15 minutes to get results, which enables healthcare providers to diagnose EV71 infection earlier allowing individuals to seek medical care sooner.
Description
EV-A71 is a common cause of hand, foot, and mouth disease. Hand, foot, and mouth disease is common in children under 5 years old, but anyone can get it. The illness is usually not serious, but it is very contagious. It spreads quickly at schools and day care centers.
Benefits
Very simple to use: The ev71 test kit only needs 3 step to get the result.
Quick result: It only takes you 10 minutes to get the enterovirus test result.
Convenient: The enterovirus 71 test kit is based on colloidal gold assay, you don't need an instrument to read the result.
Product Specifications
| Method | Colloidal Gold |
| Sample Type | Whole blood/Serum/Plasma |
| Time to Result | 10~15mins |
Storage | 2~30 ℃/36~86℉ |
Shelf Life | 24 months from date of manufacture |
Kit Size | 1/5/20/25 tests |
※ Refer to Package Insert for additional product information.
Product Performance
WIZ Results | Test result of Reference reagent | Positive coincidence rate: 99.09% (95%C.I. 95.03%~99.84%) Negative coincidence rate: 98.92% (95%C.I.96.14%~99.70%) Total coincidence rate: 98.98% (95%C.I.97.05%~99.65%) | ||
| Positive | Negative | Total | ||
| Positive | 109 | 2 | 111 | |
| Negative | 1 | 183 | 184 | |
| Total | 110 | 185 | 295 | |
Applications
Outpatient Emergency Laboratory
Clinical Departments
Community Hospital
Laboratory Departments
Health Management Center
Clinic
Certifications