Stomach H Pylori Antigen Detection Kit Machine Use

- Wizbiotech
- CE,UKCA
- China
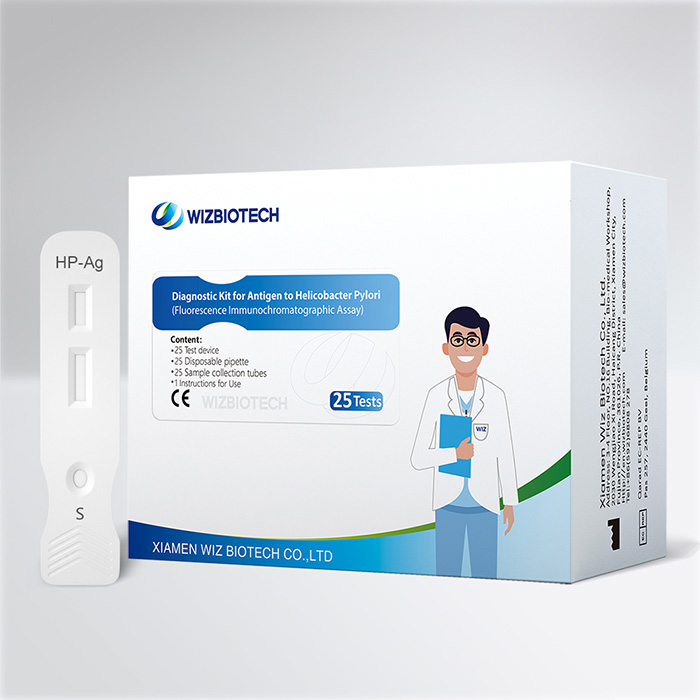
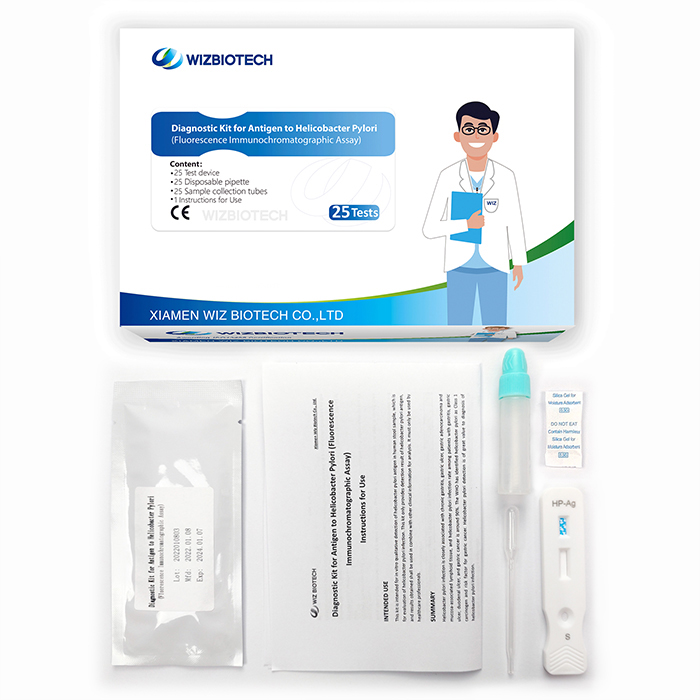
This kit is intended for in vitro qualitative detection of helicobacter pylori antigen in human stool sample. It only takes 15 minutes to get results, which enables healthcare providers to diagnose helicobacter pylori infection earlier allowing individuals to seek medical care sooner.
Description
Helicobacter pylori (H.pylori) infection is closely associated with chronic gastritis, gastric ulcer, gastric adenocarcinoma and gastric mucosa related lymphoma, and H.pylori infection rate in patients with chronic gastritis, gastric ulcer, duodenal ulcer and gastric cancer is around 90%. WHO has listed H.pylori as Class I carcinogen, and identified it as risk factor of gastric cancer. H.pylori detection is an important approach for diagnosis of H.pylori infection. From clinical perspective, existence of antibody to helicobacter pylori in patient's blood can be used as basis for auxiliary diagnosis of HP infection, and disease can be diagnosed in consideration of gastroscopy result and clinical symptoms to facilitate early treatment.
Benefits
Accurate diagnosis: H. pylori stool antigen test is highly accurate in diagnosing the presence of H. pylori bacteria in the digestive system.
Non-invasive: This test does not require any invasive procedures such as endoscopy or biopsy, making it a less stressful and more comfortable option for patients.
Monitoring treatment: H. pylori stool antigen test can be used to monitor the effectiveness of treatment for H. pylori infection.
Reduced risk of complications: Early detection and treatment of H. pylori infection can help prevent complications such as stomach ulcers, gastritis, and even stomach cancer.
Product Specifications
Method | Fluorescence Immunochromatographic Assay |
Sample Type | Faeces |
| Time to Result | 15mins |
Storage | 2~30 ℃/36~86℉ |
| Shelf Life | 24 months from date of manufacture |
| Kit Size | 1/5/20/25 tests |
※ Refer to Package Insert for additional product information.
Product Performance
WIZ Results | Test result of Reference reagent | Positive coincidence rate: 98.82% (95%C.I. 95.81%~99.68%) Negative coincidence rate: 100.00% (95%C.I. 97.04%~100.00%) Total coincidence rate: 99.32% (95%C.I. 97.57%~99.81%) | ||
| Positive | Negative | Total | ||
| Positive | 168 | 0 | 168 | |
| Negative | 2 | 126 | 128 | |
| Total | 170 | 126 | 296 | |
Applications
Outpatient Emergency Laboratory
Clinical Departments
Community Hospital
Laboratory Departments
Health Management Center
Clinic
Certifications