Blood Serum Plasma Pepsinogen I Pepsinogen Ⅱ Test For Stomach Cancer

- Wizbiotech
- CE, MDA
- China
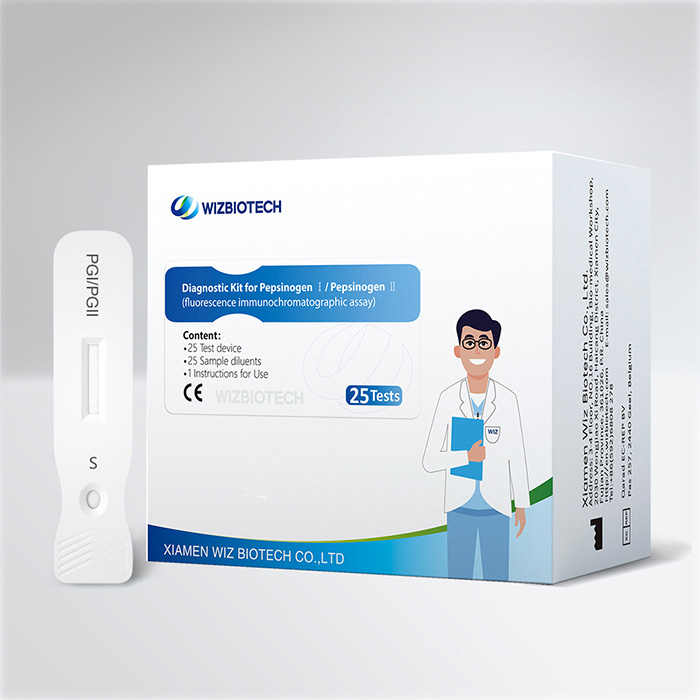
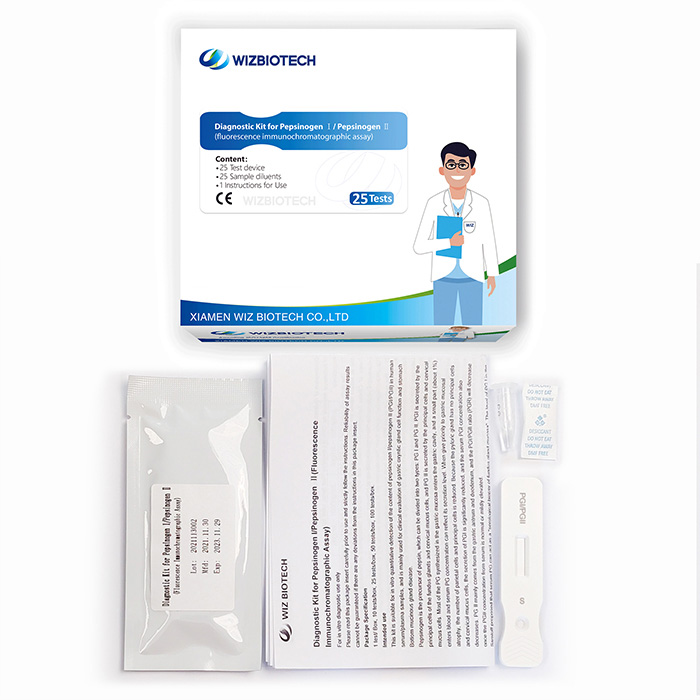
This kit is intended for the in vitro quantitative detection the content of Pepsinogen I/ Pepsinogen II (PG I/PG II) in human serum/plasma/whole blood samples to evaluate the function of gastric oxyntic gland cells and the lesion of gastric fundic mucosa. It must only be used by healthcare professionals.
Description
Pepsinogen is the precursor of pepsin and can be classified into PG I and PG II. PG I is secreted by chief cells of gastric fundus gland and mucous neck cells. PG II is secreted by chief cells and mucous neck cells. PG synthesized by gastric mucosa mostly enters gastric cavity. Only a small amount (about 1%) enters the blood. Serum PG concentration can reflect its secretion level. When mucosal atrophy of gastric body is predominant, the quantity of parietal cells and chief cells decreases. Pyloric gland has no chief cell and mucous neck cell. Therefore, PG I secretion significantly decreases and serum PG I concentration also declines. PG II is mainly originated from gastric antrum and duodenum. Serum PG II concentration is normal or increases slightly. PG I/PG II ratio (PGR) is a clinical commonly used serological diagnosis and treatment indicator and can reflect the changes of gastric status. Therefore, determination of serum PG I and PG II can assist diagnosis of early gastric diseases.
Benefits
Non-invasive: The pepsinogen test is a non-invasive method of testing for gastric function disorder. It does not require an endoscopy or other invasive procedures, which reduces patient discomfort and risk of complications.
Early detection: Early detection of gastritis, H. pylori infection, and gastric cancer.
Easy operation: The serum pepsinogen test support blood sample, which makes the test easier to perform.
Product Specifications
Method | Fluorescence Immunochromatographic Assay |
| Sample Type | Serum/Plasma/Whole blood |
Time to Result | 15mins |
| Storage | 2~30 ℃/36~86℉ |
| Shelf Life | 24 months from date of manufacture |
| Kit Size | 1/5/20/25 tests |
※ Refer to Package Insert for additional product information.
Product Performance
| Reference method vs PGI/PGII | |
| Correlation with ELISA | PGI(Y=0.985X+1.715,R=0.9838);PGII(Y=1.002X+0.018,R=0.9850) |
| CV | ≤15% |
| Differ | Within 15% |
Applications
Outpatient Emergency Laboratory
Clinical Departments
Community Hospital
Laboratory Departments
Health Management Center
Clinic
Certifications