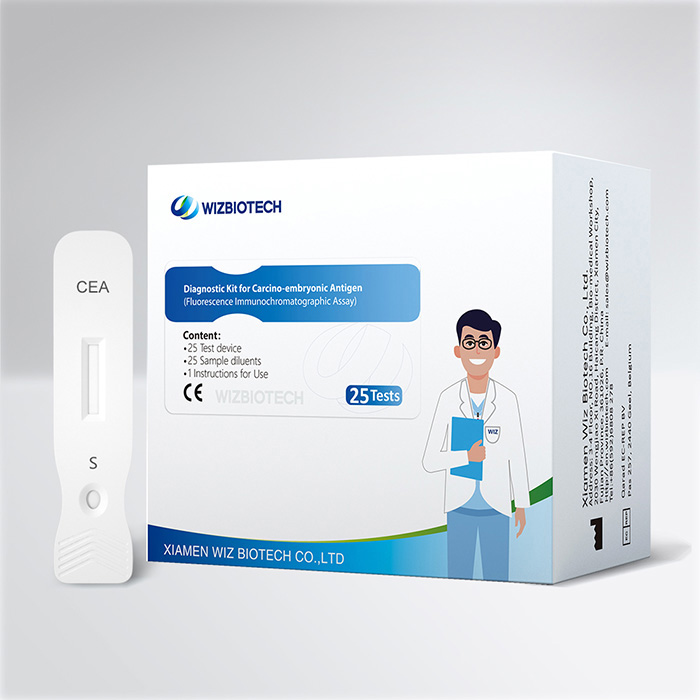
Colorectal Cancer Treatment Surveillance CEA Carcino-Embryonic Antigen Rapid Detection Kit

- Wizbiotech
- CE,UKCA
- China
The diagnostic kit of carcino-embryonic antigen is used to detect the presence of cancer antigens in the body, as well as to monitor the progression of the disease.The operation is simple and fast, and the test result can be obtained in 15 minutes.
Introduction
Carcino-embryonic antigen is a broad-spectrum tumor marker, which can indicate existence of different tumors to people. It’s an excellent tumor marker for therapeutic effect judgment, disease progress, monitoring and prognostic estimation of colorectal cancer, breast carcinoma and lung cancer.This kit uses double-antibody sandwich assay and fluorescence immunochromatographic assay of high specificity for quantitative detection of carcino-embryonic antigen (CEA) in human serum/plasma/whole blood sample.
Product Specifications
Method | Fluorescence Immunochromatographic Assay |
| Sample Type | Serum/Plasma/Whole blood |
Time to Result | 15mins |
| Storage | 2~30 ℃/36~86℉ |
Shelf Life | 24 months from date of manufacture |
Kit Size | 1/5/20/25 tests |
※ Refer to Package Insert for additional product information.
Product Performance
| Reference method vs CEA | |
| Correlation with electrochemiluminescence | Y=0.940X+2.249 and R=0.9889 |
| CV | ≤15% |
| Differ | Within 15% |
Applications
Outpatient Emergency Laboratory
Clinical Departments
Community Hospital
Laboratory Departments
Health Management Center
Clinic
Certifications