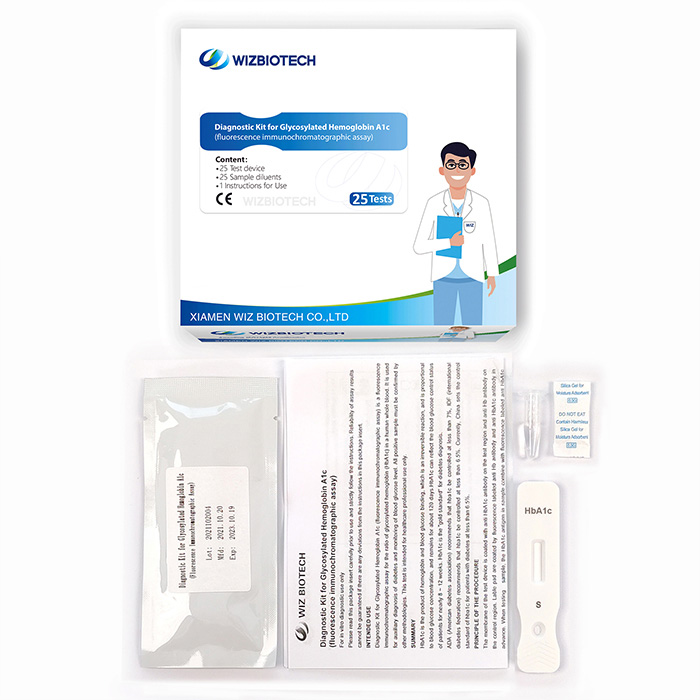
HbA1C Glycosylated Hemoglobin A1C Level Blood Sugard Test Diabetes Risk Management Test

- Wizbiotech
- CE, UKCA, MDA
- China
This kit is applicable to the in vitro quantitative detection on the content of glycated hemoglobin (HbA1c) in human whole blood samples and is mainly used for implementing auxiliary diagnosis of diabetes and monitor blood glucose level.
Benefits
Easy to operate.
Rapid, result in 15 mins.15 minutes, to avoid the long examination time caused by patients repeatedly leave for medical treatment.
Result interpreted by machine.The user experience is better and there is less chance of human error.
Clinical Significance
Assess the overall control of blood sugar in patients in the past 2-3months
Predict the risk of chronic complications of diabetes
Diagnostic indicators of diabetes
Differential diagnosis of “stress hyperglycemia”
Product Specifications
Method | Fluorescence Immunochromatographic Assay |
| Sample Type | whole blood |
| Time to Result | 15mins |
Storage | 2~30 ℃/36~86℉ |
| Shelf Life | 24 months from date of manufacture |
| Kit Size | 1/5/20/25 tests |
※ Refer to Package Insert for additional product information.
Product Performance
Reference method vs HbA1C | |
Correlation with HPLC | Y=0.960X+0.277,R=0.9849 |
CV | ≤10% |
Differ | Within 10% |
Application
Outpatient Emergency Laboratory
Clinical Departments
Community Hospital
Laboratory Departments
Health Management Center
Clinic
Certifications