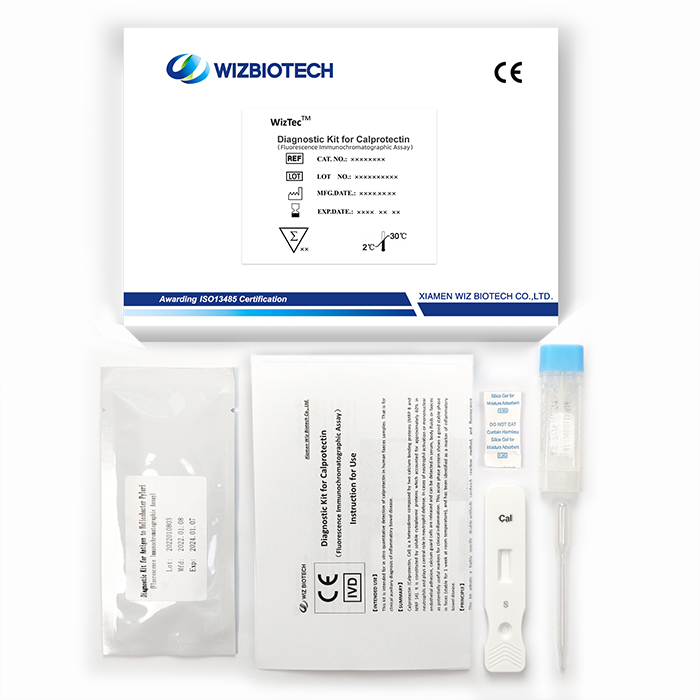
Lab Calprotectin Test For Abdominal Pain Ibd Monitor Crohn'S Disease Ulcerative Colitis

- Wizbiotech
- CE
- China
The kit is intended for in vitro quantitative detection of calprotectin in human faeces samples. The calprotectin test result can be obtained in 15 mins and is for clinical auxiliary diagnosis of inflammatory bowel disease.
Description
Calprotectin is a protein expressed by neutrophils. The presence of fecal calprotectin is a sensitive indicator of gastrointestinal inflammation, with higher levels representing more inflammation. The U.S. Food and Drug Administration has approved the use of fecal calprotectin testing to aid in the diagnosis of inflammatory bowel disease (IBD) in adults and children presenting with gastrointestinal symptoms, and differentiating IBD from irritable bowel syndrome.
Benefits
Disease monitoring: The fecal calprotectin quantitative test helps in monitoring the effectiveness of treatment and detecting any recurrence of the disease.
Diagnstic value: The test result can differentiate between IBD and IBS.
Non-invasive: The calprotectin test takes stool as sample, which is a non-invasive choice for patient.
Product Specifications
Method | Fluorescence Immunochromatographic Assay |
Sample Type | Faeces |
| Time to Result | 15mins |
Storage | 2~30 ℃/36~86℉ |
Shelf Life | 18 months from date of manufacture |
Kit Size | 1/2/5/10/25/50 tests |
※ Refer to Package Insert for additional product information.
Product Performance
Wiz Biotech | Reference reagent results | PPA:97.59% (C.I. 91.63%~99.34%) NPA:100.00% (C.I. 96.82%~100.00%) OPA:99.00% (C.I. 96.43%~99.73%) | ||
| Positive | Negative | Total | ||
| Positive | 81 | 0 | 81 | |
| Negative | 2 | 117 | 119 | |
| Total | 83 | 117 | 200 | |
Applications
Outpatient Emergency Laboratory
Clinical Departments
Community Hospital
Laboratory Departments
Health Management Center
Clinic
Certification