Fecal Occult Blood Colon Fit Fecal Immunoassay Test Before Colonoscopy

- Wizbiotech
- CE,UKCA
- China
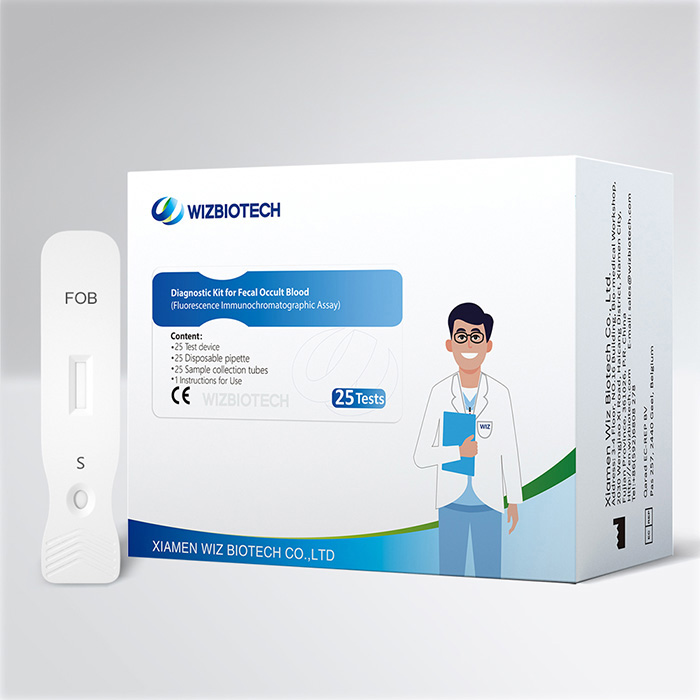
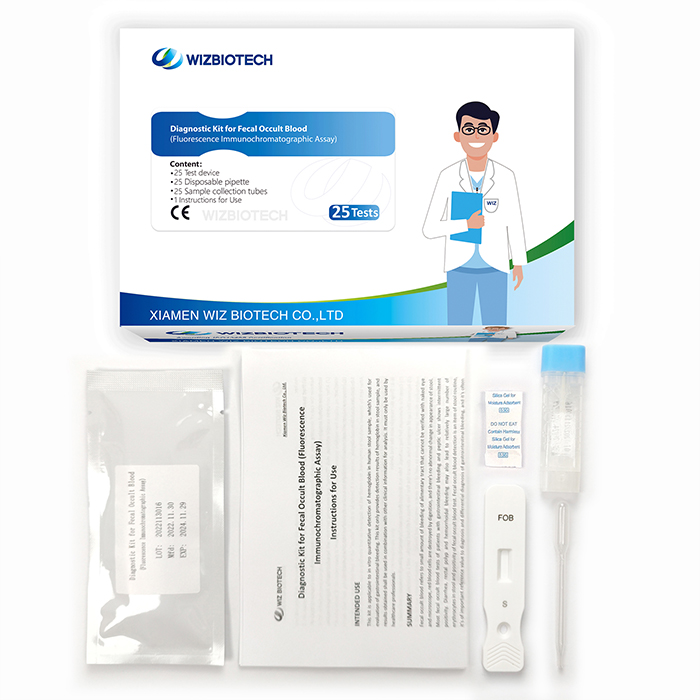
The fobt test kit is applicable to in vitro quantitative detection of hemoglobin in human stool sample, which's used for evaluation of gastrointestinal bleeding. It must only be used by healthcare professionals.
Description
Fecal occult blood refers to small amount of bleeding of alimentary tract that cannot be verified with naked eye and microscope, red blood cells are destroyed by digestion, and there's no abnormal change in appearance of stool. Most fecal occult blood tests of patients with gastrointestinal bleeding and peptic ulcer shows intermittent positivity. Diarrhea, rectal polyp and hemorrhoidal bleeding may also lead to relatively large number of erythrocytes in stool and positivity of fecal occult blood test. Fecal occult blood detection is an item of stool routine, it’s of important reference value to diagnosis and differential diagnosis of gastrointestinal bleeding, and it's often used as a screening indicator for diagnosis of gastrointestinal bleeding.
Benefits
Early detection of colon cancer: Quantitative fecal occult blood tests can detect small amounts of blood in the stool, which could indicate the presence of colon cancer or precancerous polyps. Early detection of these conditions can improve treatment outcomes and increase the chances of survival.
Screening for other gastrointestinal conditions: Besides colon cancer, quantitative fecal occult blood tests can also detect other gastrointestinal conditions such as inflammatory bowel disease, diverticulitis, and hemorrhoids.
Non-invasive: Quantitative fecal occult blood tests are less invasive option compared to other colon cancer screening methods such as colonoscopy.
Reducing health care costs: Early detection of cancer or other gastrointestinal conditions can reduce healthcare costs by avoiding the need for more invasive and expensive procedures.
Product Specifications
Method | Fluorescence Immunochromatographic Assay |
| Sample Type | Faeces |
| Time to Result | 15mins |
Storage | 2~30 ℃/36~86℉ |
| Shelf Life | 24 months from date of manufacture |
| Kit Size | 1/5/20/25 tests |
※ Refer to Package Insert for additional product information.
Product Performance
| Reference method vs Fob | |
| Correlation with ELISA | Y=0.980x +3.680 , R=0.9808 |
| CV | ≤15% |
| Differ | Within 15% |
Applications
Outpatient Emergency Laboratory
Clinical Departments
Community Hospital
Laboratory Departments
Health Management Center
Clinic
Certifications